You are here
AstraZeneca US trials thrown into doubt after promising data
By AFP - Mar 23,2021 - Last updated at Mar 23,2021
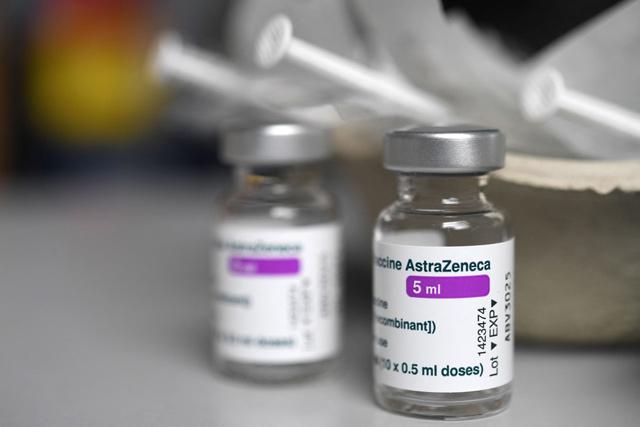
This file photo taken on March 12, shows empty vials of the AstraZeneca COVID-19 vaccine at a vaccination centre at the Universite Bretagne Occidentale in Brest, western France (AFP photo)
WASHINGTON/ BEIJING — A US health agency raised concerns Tuesday that AstraZeneca may have included out-of-date information during trials of its COVID-19 vaccine, a day after the company said its drug was highly effective in preventing the disease.
The Anglo-Swedish pharma giant stood by its assessment of the results of its US trials, saying it would publish new data "within 48 hours" in response to concerns raised by the US National Institute for Allergies and Infectious Diseases.
The fresh problems for the drugmaker, which had on Monday hailed its vaccine as 79 per cent effective at preventing COVID-19, come as Europe continues to wrangle over shortages of the jab and after weeks of safety concerns.
With AstraZeneca delivering only 30 per cent of the doses it promised the EU for the first quarter, Germany threw its weight behind a ban on European exports of the jab, while also announcing strict virus measures over Easter to contain spiralling infections.
Meanwhile, Britain marked the anniversary of its first coronavirus lockdown by holding a minute's silence for the more than 126,000 of its citizens who have died from COVID-19, the fifth-highest death toll in the world.
The global death toll now stands at more than 2.7 million.
AstraZeneca 'problem'
AstraZeneca's vaccine had been hailed as a potential game-changer in the fight against the pandemic as it is cheaper and easier to store than many of its rivals, making it more accessible for poorer nations.
But public confidence in the drug has tumbled after more than a dozen countries temporarily suspended its rollout because of isolated cases of blood clots in people who had received a dose.
The EU’s medicines regulator and the World Health Organisation insist there is no evidence linking the vaccine to blood clots, and none was found in the large-scale trials in the US.
Scant supplies of AstraZeneca doses in Europe have meanwhile hampered the continent’s vaccine rollouts, which lag far behind countries like the UK and Israel in their pace.
French President Emmanuel Macron said Tuesday that his country should be giving vaccines “morning, noon and evening”, as his government announced that 35 new mass inoculation centres would open soon.
“We’re going to change pace from April,” he said, adding that there should be “no weekend and days off when it comes to vaccinations”.
Meanwhile, China’s drug authority has approved for clinical trials an inhaled COVID-19 vaccine co-developed by domestic firm CanSino Biologics, the company said in a filing on Tuesday.
The move comes after the National Medical Products Administration gave another CanSino vaccine conditional approval last month, allowing it for public use.
China currently has five coronavirus vaccines that have been given conditional market approval or allowed for emergency use, but none of these are administered by inhalation.
CanSino said in its latest filing on the Hong Kong stock exchange that the vaccine for inhalation was jointly developed by the company and the Beijing Institute of Biotechnology, adding that their clinical trial application got the green light on Monday.
But it warned that the vaccine’s safety and efficacy remain “subject to confirmation” in trials.
As of March 20, China had administered 74.96 million coronavirus vaccine doses, according to Our World in Data, a collaboration between Oxford University and a charity.
Chinese embassies in some countries including the United States, Australia and India have issued notices saying the country will open visa applications to select people who have taken a China-made jab.
On Tuesday, a Chinese foreign ministry official said it is in “close communication” with various countries and “willing to reach mutually beneficial arrangements” to facilitate cross-border travel.
‘Much deadlier’
In Germany, Merkel ordered a nationwide shutdown for five days between April 1 and April 5 as Christians celebrate Easter, with almost all shops closed and religious services moved online.
“The situation is serious,” Merkel said. “Case numbers are rising exponentially and intensive care beds are filling up again.”
A variant first identified in Britain has become the dominant strain circulating in Germany, she said, adding: “We are in a new pandemic.”
“Essentially, we have a new virus... it is much deadlier, much more infectious and infectious for much longer.”
Elsewhere, Ukraine said it too was battling a new wave of infections as it reported the highest death toll since the beginning of the crisis, with 300 fatalities in the past 24 hours.
Banksy fundraiser
In tribute to its COVID dead, Britain held a “National Day of Reflection” on Tuesday, with parliament holding a minute’s silence and bells ringing out across the country.
Prime Minister Boris Johnson said the anniversary of Britain’s first lockdown was “an opportunity to reflect on the past year — one of the most difficult in our country’s history”.
The country’s state-run National Health Service has been under huge strain, and on Tuesday a painting by UK street artist Banksy sold for a record £16.75 million ($23.1 million, 19.4 million euros) in an auction by Christie’s to raise money for health workers.
The artist left a note with the piece thanking hospital staff for their work battling the pandemic.
“Thanks for all you’re doing. I hope this brightens up the place a bit, even if it’s only in black and white,” he wrote.
Related Articles
LONDON — Queen Elizabeth II’s oldest son and heir on Wednesday criticised opposition to coronavirus vaccines, as British ministers sought to
WASHINGTON — A decision on whether to end a US pause in vaccinations with the Johnson & Johnson COVID-19 shot is likely by Friday, top U
GENEVA — The EU's medicines regulator said onTuesday there was so far "no indication" the AstraZeneca vaccine causes blood clots, urging cou